Explosion characteristics of syngas mixtures at elevated temperatures and pressures
Výbuchové charakteristiky syntetického plynu za zvýšených počátečních teplot a tlaků
Jan SKŘÍNSKÝ1, Ján VEREŠ1, Václav PEER1, Pavel FRIEDEL1
[1]Energy Research Centre, VSB-Technical University of Ostrava, corresponding author: jan.skrinsky@vsb.cz
Abstract
Renewable energies became more and more important in the last years. The production of biogas using agricultural waste and the use of wind and solar energy in combination with water electrolysis is one way to substitute natural gas. Therefore the number of syngas plants is growing very fast. On the other hand, the operation of such plants could be responsible for a significant number of accidents. The main focuses of this contribution are the explosion characteristics and hazards arising from the biogas. Primarily, these are the hazards of fire and explosion induced by flammable components of syngas. However, further hazards are the dangers of asphyxiation and poisoning by gases such as carbon monooxide. These hazards will be the aim of the following article. In order to prevent explosions when storing and handling syngas it is necessary to know the explosion limits of individual gas components and its gas mixtures in mixture with air. However, syngas from gasification unit can vary significantly in its composition. Therefore, for each gas composition the explosion limits would have to be determined. This would require a considerable amount of time and effort. Due to this fact, the explosion limits of syngas are frequently referred to only by the hydrogen fraction of the gas mixture in the safety-relevant literature. In reality as syngas consists of hydrogen, methane, carbon monoxide, carbon dioxide and further residual gases the explosion limits are generally over or underestimated.
Keywords: Maximum Explosion Pressure; Constant Volume Adiabatic Temperature; Syngas
Abstrakt
Obnovitelné zdroje energie se v posledních letech stávají stále více a více důležité. Výroba bioplynu využívající zemědělského odpadu a využívání větrné a solární energie v kombinaci s elektrolýzou vody, je možný způsob, jak snížit spotřebu zemního plynu. Proto velmi rychle narůstá počet zařízení k výrobě syntézního plynu. Na druhou stranu, může být provoz těchto zařízení zodpovědný za velký počet havárií. Hlavním cílem tohoto příspěvku jsou výbuchové charakteristiky a nebezpečí související s používáním bioplynu. Primárně se jedná o nebezpečí vzniku požáru a výbuchu hořlavých složek syntetického plynu. Nicméně existují i další nebezpečí jako udušení a otrava plyny, jako je oxid uhelnatý. Tato nebezpečí budou předmětem následujícího článku. Aby bylo možné zabránit výbuchům při skladování a manipulaci se syntetickým plynem, je nutné znát limity výbušnosti jednotlivých složek plynů a plynných směsí tohoto plynu ve směsi se vzduchem. Nicméně, složení syntézního plynu ze zplyňovací jednotky se může výrazně lišit. Proto je pro každé složení plynu nutné znát meze výbušnosti, které je nutné experimentálně stanovit. To by vyžadovalo značné množství času a financí. Vzhledem k této skutečnosti se výbuchové charakteristiky syntetického plynu často stanovují pouze z výbuchových charakteristik frakce vodíku v plynné směsi, jak lze dohledat v relevantní literatuře. Ve skutečnosti je směs syntetického plynu složena z vodíku, metanu, oxidu uhelnatého, oxidu uhličitého a dalších zbytkových plynů a výbuchové charakteristiky jsou obecně nižší nebo vyšší než skutečné.
Klíčová slova: Maximální výbuchový tlak; Adiabatická teplota při konstantním objemu; Syntetický plyn
1. Introduction
Biomass gasification is the thermal conversion of a heterogeneous solid material into a gaseous intermediate fuel, syngas, consisting primarily of carbon monoxide and hydrogen that can be used for the production of heat, power, liquid fuels, and chemicals. Biomass is the only renewable source of organic carbon, and its use is a key issue of sustainable development. The gasification of biomass has the potential to offer a major contribution to meeting the international targets for CO2 mitigation (Knoef et al., 2012).
Gasification is also called “staged combustion”, since usually the syngas is produced with the intent to burn it later. This raises the question why first gasifying and then burning the gas over direct combustion of the biomass. There are several essential advantages: possibility to transport in pipelines; ease of control and continuous operation; clean combustion of produced gas since impurities can be removed from the producer gas, and the volume of producer gas is much smaller compared to flue gas; efficient and clean combustion since the exact required air can be mixed for optimum combustion; producer gas can be used in engines or turbines with higher efficiencies over steam devices; producer gas, in particularly syngas, can be used for chemical synthesis like fertilizers and transportation fuels (Reed and Guar, 2000).
The characteristic safety parameter of syngas from biomass gasification in a closed vessel explosion, so called explosion characteristic, discussed in this contribution is the maximum explosion pressure. The maximum explosion pressure is the highest explosion pressure over the flammable range in a closed volume at a given fuel concentration (Eckhoff, 2005).
These explosion characteristics are important for design of safety devices (e.g. relief systems, vents), able to ensure active protection of pressure vessels where flammable mixtures are formed. Beyond safety devices, the values of these parameters are useful for emergency planning especially for developing scenarios where emergency relief or external heat transfer may be inadequate. At the same time, the maximum explosion pressure that the explosion reaction can generate is one good measure of the magnitude of the hazard associated with the reaction (CCPS, 1995).
The present contribution presents absolute explosion pressures (in terms of bar(a)) of stoichiometric H2/CH4/CO/C3H8/CO2/O2/N2 syngas mixtures with air calculated for various initial temperatures and pressures. The aim of this contribution is to evaluate the influence of the temperature on the explosion parameters, namely maximum explosion pressure, of the syngas produced by generator during autothermal gasification in fixed-bed. The primary outcomes are: explosion parameters at ambient conditions and elevated temperatures and pressures.
2. Basic description of technology
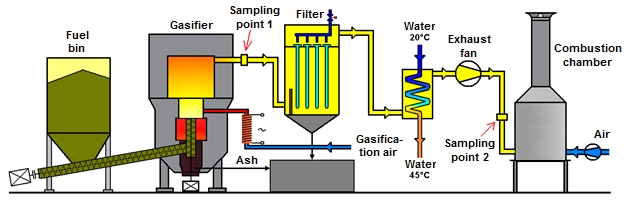
The autothermal gasification technology at the Energy Research Center was developed as a result of the Project “Biomass Gasification for Cogeneration“. The aim of the Project was using the generated gas in a cogeneration unit with a piston combustion engine for the electric energy and heat production. Base of this technology is a fixed-bed gasification generator. The fuel is supplied to the grate of the gasification reactor from a fuel bin by the two screw conveyors. The fuel is supplied to the reactor´s lower part automatically based on a measured temperature in the gasification reactor. The generated gas is transported to the hot filter, where are removed dust particles. Behind the filter are gas cooler, exhaust fan and combustion chamber, in which the producer gas is combusted.
a)
b)
Figure 1: Autothermal gasification technology: a) gas cleaning devices - hot filter, gas cooling and exhaust fan; b) fuel bin and fuel transport into autothermal gasifier
The technology is operated in the low under pressure and it is provided by a necessary measurement and control technological units. Thermal output of the autothermal gasifier is 100 kW. As a fuel for testing was used wood pellets with diameter 6 mm. Gas for analysis was sampled from sampling point 2 depicted in Figure 2.
Figure 2: Technological scheme of autothermal gasification unit
The mixture composition investigated in this contribution is given in Table 1.
| Ingredients | H2 | CH4 | CO | C3H8 | CO2 | O2 | N2 |
|---|---|---|---|---|---|---|---|
| Content vol. % | 15.85 | 2.10 | 20.15 | 0.99 | 11.83 | 0.12 | 48.96 |
Table 1: The compositions of syngas for analysis
Due to the complexity of the involved physical phenomena and to the lack of an adequate amount of reliable experimental data, a very limited number of different models and calculation procedures for estimating the physical consequences following the explosion of a gaseous state mixture as syngas are presently reported in the literature. The following model was used to investigate and quantify the role of initial temperature and pressure in affecting the explosion behavior of syngas mixture.
3. Previous studies and analysis
Mathematical model used in this study computes adiabatic flame temperatures and constant volume adiabatic explosion pressures at various initial temperatures and pressures, taking into account 26 species. It has been shown (Skrinsky et al., 2015) that the model is able to predict, with a reasonable accuracy in different fuel-enriched conditions, for different types of gaseous mixtures explosions.
Element balanced equation:
![]() (1)
(1)
where ajare moles of j-th element (atom); niare moles of i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; j is the number of species.
The free energy (Helmholtz function) of the system F equation:
![]() (2)
(2)
where F is the free energy (Helmholtz function) of the system; ni are moles of i-th species; fi is free energy of i-th species.
![]() (3)
(3)
where fi is free energy of i-th species; λi are the Lagrangian multipliers for each element (j=1 to J); Ai,jnumber of j-th element in i-th species.
![]() (4)
(4)
where gi is the Gibbs free energy for single i-th species; ΔHfis the enthalpy of formation of the i-th species at standard conditions;
![]() (5)
(5)
where giis the Gibbs free energy for single i-th species; R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
![]() (6)
(6)
where ajare moles of j-th element (atom); niare moles of i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; j is the number of species; gi is the Gibbs free energy for single i-th species; λi are the Lagrangian multipliers for each element (j=1 to J); R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
![]() (7)
(7)
where gi is the Gibbs free energy for single i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; λi are the Lagrangian multipliers for each element (j=1 to J); R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
The equations 1-7 were used to investigate and quantify the role of initial temperature and pressure in affecting the explosion behaviour of syngas-air mixtures.
4. Results and discussions
Results of explosion experiments depend on many different parameters of the investigated process, such as the energy and type of ignition source, size and shape of explosion chamber, initial temperature, initial pressure and composition of the flammable mixture. To ensure the compatibility of data we selected the results for experiments that are in agreement with EN 13673-1. The results of experiments are summarized in Table 2.
A) Experimental and theoretical Pmax for H2/CH4/CO/CO2/O2/N2 at ambient temperature and pressure
| Characteristic | Abbreviation | Unit | H2 | CH4 | CO | C3H8 |
|---|---|---|---|---|---|---|
| Maximum explosion pressure | Pmax | bar(a) | 8.3G | 8.1G | 8.2G | 9.4G |
| 8.2EN | 8.3EN | 8.3CO | 9.1MI | |||
| 7.9HO | 8.7RA | 8.0D | 8.7MO |
Table 2: Safety characteristics of flammable syngas components
G = GESTIS-Substance database (IFA); EN = standard EN 13673-1; CO = Compendium of Environmental Standards (CES); MI = Mitu et al., 2011; HO = Hochtappels et al., 1963; RA = Razus et al., 2006; D = Design Institute for Physical Properties (AiChE); MO = Movileanu et al., 2011.
Table 2 compares the theoretically derived data for the maximum explosion pressure of the studied syngas mixture components. The values were adopted from the databases as the IFA and the AiChE, and from the literature (Mitu et al., 2011; Hochtappels et al., 1963; Razus et al., 2006; AiChE; Movileanu et al., 2011; standard EN 13673-1). In Table 2, for example the values of the Pmax for adopted from standard EN 13673-1 and Hochtappels et al., 1963 (8.2 bar(a) and 7.9 bar(a), respectively) clearly appear to be quite different. Similarly, looking for C3H8 (8.7 bar(a) and 9.4 bar(a)).
B) Teoretical Pmax for H2/CH4/CO/C3H8/CO2/O2/N2 at elevated temperature and pressure
Computed adiabatic temperatures, Tf, and maximum explosion pressures, pmax, for syngas-air mixtures (4.80 vol. %, 10.50 vol. %, 15.50 vol. %) at various initial temperatures, Tinit, and ambient initial pressure are given in Table 3.
|
Fuel fraction [vol.%] |
Initial temperature (K) |
|||
|---|---|---|---|---|
| 298 | 358 | 418 | 478 | |
| 10.0 | 3.04 | 2.67 | 2.40 | 2.21 |
| 35.0 | 7.39 | 6.23 | 5.40 | 4.77 |
| 60.0 | 7.74 | 6.54 | 5.68 | 5.04 |
| 85.0 | 4.06 | 3.42 | 2.97 | 2.66 |
Table 3: Computed explosion pressures for H2/CH4/CO/C3H8/CO2/O2/N2 mixtures at p0 = 1 bar(a)
From the numerical results of Table 3 it is possible to identify that the increase in the initial temperature lowers the maximum explosion pressure, and increases the flammability range. The value of the explosion pressure with varying H2/CH4/CO/C3H8/CO2/O2/N2 concentration is similar at all investigated initial temperatures. The maximum value of the explosion pressure is found close to 48 vol. % of syngas for all conditions. Further, in Table 4, we reported simulations on the explosion properties of H2/CH4/CO/C3H8/CO2/O2/N2 mixtures at various initial pressure and ambient initial temperature.
|
Fuel fraction [vol.%] |
Initial pressure (bar(a)) |
|||
|---|---|---|---|---|
| 1 | 5 | 10 | 15 | |
| 10.0 | 3.23 | 16.15 | 32.29 | 48.44 |
| 35.0 | 7.53 | 38.03 | 76.29 | 114.60 |
| 60.0 | 7.64 | 38.30 | 76.65 | 115.01 |
| 85.0 | 4.01 | 21.36 | 43.88 | 66.85 |
Table 4: Computed explosion pressures for H2/CH4/CO/C3H8/CO2/O2/N2 mixtures with air at T0 = 298 K
From the data performed in Table 4, it is clear that the increase of initial pressure has significant effect on explosion pressure when only the air served as oxidant. This is a good approximation at initial pressures i.e. up to 15 bar(a), but we may assume that will be increasing wrong at higher pressures.
a)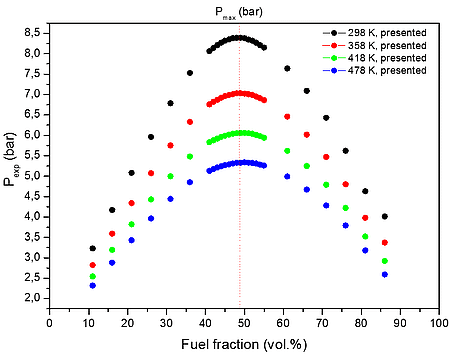
b)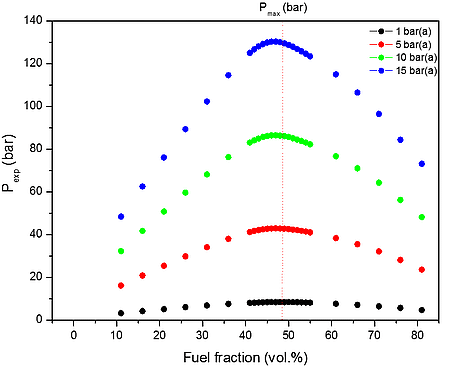
Figure 1: Calculated explosion pressure vs fuel fraction for explosions of a) H2/CH4/CO/C3H8/CO2/O2/N2 mixture with air at 298 K (top), 358 K (upper middle), 418 K (lower middle), and 478 K (bottom); b) H2/CH4/CO/C3H8/CO2/O2/N2 with air mixture at 1 bar(a) (top), 5 bar(a) (upper middle), 10 bar(a) (lower middle), and 15 bar(a) (bottom)
a)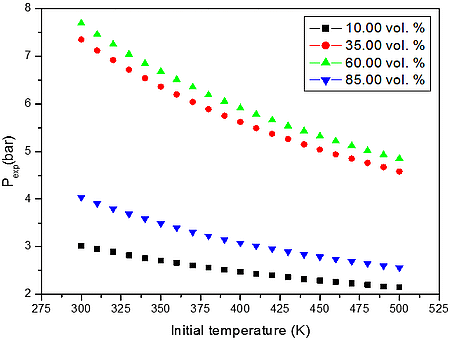
b)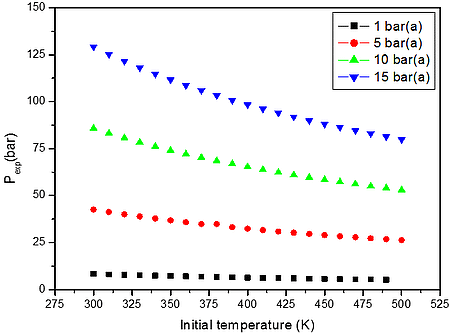
Figure 2: Calculated explosion pressure vs initial temperature for explosions of a) H2/CH4/CO/C3H8/CO2/O2/N2 mixture with air at 10.00 vol. % (top), 35.00 vol. % (upper middle), 60.00 vol. % (lower middle), and 85.00 vol. % (bottom); b) H2/CH4/CO/C3H8/CO2/O2/N2 mixture with air at 1 bar(a) (top), 5 bar(a) (upper middle), 10 bar(a) (lower middle), and 15 bar(a) (bottom)
C) Teoretical effects of syngas explosion at ambient and elevated pressures and temperatures
The destructions on structures such as described in Table 5, showing pressure levels and corresponding damages, have been established from tests or actual accidental explosions. The data in Table 5 provide only a small sample on information from various sources. A closer study shows that the greatest part leads us back to information taken out of reference (GreenBook, 1997) in which primarily the consequences of nuclear explosions have been investigated. The damages shown in Table 5 correspond to the pressures given in this table. From these data it is possible to obtain a rough idea about the damages which could be expected. The quantity used for the explosion damage decription is explosion pressure.
| Description of damage |
Explosion pressure bar(a)×10-2 |
|---|---|
| Connections between steel or aluminium plates have failed | 7-14 |
| Minor damage to steel frames | 8-10 |
| Walls made of concrete blocks have collapsed | 15-20 |
| Collapsad of steel frames and displacement of foundation | 20 |
| Industrial steel self-framing structure collapsed | 20-30 |
| Cladding of light industry building ripped-off | 30 |
| Brickstones walls, 20-30 cm, have collapsed | 50 |
| Displacement of a cylindrical storage tank, failure of pipes | 50-100 |
Table 5: The consequences of explosion effects on structures from empirical data
5. Conclusion
The adiabatic explosion pressures H2/CH4/CO/C3H8/CO2/O2/N2 mixture with air at various initial temperatures and pressures were calculated. The model predictions for the syngas mixtures are compared for four different initial temperatures. Although the results from the evaluation indicate that presented theoretical simulations can become a valuable tool for rough estimation, the modeling requires further improvements to be useful for consequence modeling and design of industrial facilities. Thus, at the first stage, the equilibrium calculations can be used as a rough calculation of a worst case scenario. At the same time, these values will be used as approximate initial values for explosion experiments carried out in heated 1 m3 explosion apparatus designed by OZM Research s.r.o. at Energy Research Centre, VŠB - Technical University of Ostrava. As the practical outcome these results will apply for the syngas produced by generator during autothermal gasification in fixed-bed. The results represents a continuation of numerous efforts by various research groups, where the key underlying problem has been the understanding of results obtained in laboratory tests for predicting the consequences of multicomponent gas mixture explosion scenarios in industry (Skřínský et al., 2014; Skřínský et al., 2015; Skřínská 1 et al., 2014; Skřínská2 et al., 2014).
6. Acknowledgement
This work would not have been possible without the financial support of two grants: (1) Innovation for Efficiency and Environment, reg. no. ED0036/01/01 supported by Operation Program Research and development for Innovation and financed by the Ministry of Education, Youth and Sports; (2) Innovation for Efficiency and Environment - Growth, reg. no. LO1403 supported by National Programme for Sustainability and financed by the Ministry of Education, Youth and Sports. J.V. is thankful for the financial support of the project “Support research and development in the Moravian-Silesian Region 2014 DT 1-International research teams” 02722/2014/RRC, financed from the budget of the Moravian-Silesian Region. J.S. is thankful to his advisors Ing. Jan Koloničný, Ph.D. and doc. Dr. Ing. Břetislav Janovský, CSc. for their knowledge support in the area of decision process, industrial explosion experiments and enthusiasm for a team work.
7. References
[1] KNOEF …[et al.]. Handbook biomass gasification: second edition. 2nd ed. BTG Biomass Technology Group BV, 2012. 509 s. ISBN 10-9081938509.
[2] REED, T.; GUAR, S. A survey of Biomass Gasification 2000: Gasifier projects and manufacturers around the world.
[3] ECKHOFF, R. Explosion Hazards in the Process Industries. 1st ed. Gulf Publishing Company, 2005. 436 s. ISBN 978-0-9765-1134-2.
[4] CCPS: Center for Chemical Process Safety. Guidelines for Safe Storage and Handling of Reactive Materials. Center for Chemical Process Safety, 1995. 364 s. ISBN 978-0-8169-0629-1.
[5] SKŘÍNSKÝ, Jan…[et al.]. Explosion characteristics of methane at elevated initial temperatures. Časopis výzkumu a aplikací v profesionální bezpečnosti [online], 2015, roč. 8, č. 2-3. Dostupný z WWW: <http://www.bozpinfo.cz/josra/josra-02-03-2015/methane-explosion-characterist.html>. ISSN 1803-3687.
[6] MITU M., BRÎNZEA V., MUSUC A., RĂZUŞ D., OANCEA D. Deflagration Parameters of Propane–Air Mixtures in a Closed Cylindrical Vessel. U.P.B. Sci. Bull., Series B, vol. 73, No. 3, 2011.
[7] HOCHTAPPELS, K. Report on experimentally determined explosion limits, explosion pressures and rates of explosion pressure rise – Part 1: Methane, Hydrogen, Propylene. Journal of Loss MOORE, W. J. Physical Chemistry (4th.ed.) Longmans Green & Co. Ltd. 1963.
[8] MOVILEANU, C.; MITU, M.; BRINZEA V. …[et al.]. Adiabatic Flame Temperature of Fuel-Air Mixtures in Isobaric and Isochoric Combustion Processes. Rev. Chim. 2011, vol. 62, No. 4, pp. 376-379.
[9] EN 13673-1. Determination of maximum explosion pressure and maximum explosion pressure rise - Part I: maximum explosion pressure. 2003.
[10] RAZUS, D.; OANCEA, D.; MOVILEANU, C. Burning velocity evaluation from pressure evolution during the early stage of closed-vessel explosion. Journal of Loss Prevention in the Process Industries, 2006, vol. 19, n. 4, pp. 334-342.
[11] TNO: methods for the determination of possible damage to pe-ople and objects resulting from release of hazardous materials: CPR 14E: Green Book. 1st ed. Committee for the Prevention of Disasters, 1992.
[12] SKŘÍNSKÁ, M. …[et al.]. BLEVE: Cases, Causes, Consequences and Prevention. Mater. Sci. Forum, 2014, vol. 811, s. 91-94.
[13] SKŘÍNSKÁ, M. …[et al.]. Mathematical models for the prediction of heat flux from fire balls. WSEAS Transactions HAMT, 2014, vol. 9, s. 243-250.
[14] SKŘÍNSKÝ, J. …[et al.]. Application of emergency planning criteria for the control of major accident hazards-calculation of the consequences of fire accidents (2014). In Safety, Reliability and Risk Analysis: Beyond the Horizon: Proceedings of the European Safety and Reliability Conference, ESREL 2013. S. 135-142.
Vzorová citace
SKŘÍNSKÝ, J. …[et al.]. explosion characteristics of syngas mixtures at elevated temperatures and pressures. Časopis výzkumu a aplikací v profesionální bezpečnosti [online], 2015, roč. 8, č. 4. Dostupný z WWW: <http://www.bozpinfo.cz/josra/josra-04-2015/explosion-characteristics.html>. ISSN 1803-3687.
Užitečné odkazy
Provozovatel portálu
Jeruzalémská 1283/9
110 00 Praha 1