Effects of Hydrogen Addition on Explosion Characteristics of Methan-air Mixture
VLIV PŘÍDAVKU VODÍKU NA VÝBUŠNÉ CHARAKTERISTIKY metanu A VZDUCHU
Kevin Daune1, Jan Skřínský2
1Département ingénieur industriel, Haute Ecole de Namur-Liège-Luxembourg, Rue Saint-Donat 130, 5002 Namur, Belgium; e-mail: kevin.daune@hotmail.fr
2Energy Research Centre, VSB-Technical University of Ostrava, 17. Listopadu 15/2172, 708 33 Ostrava, Czech Republic; e-mail: jan.skrinsky@vsb.cz
Abstract
This data is very interesting because previous studies are published only for pure gases. The data obtained can be very useful for determining the safety precautions of equipment and technologies with different concentrations of pure gases, mixtures with different stoichiometric ratios and even different starting temperatures (also very important in industry). For future studies, we propose the determination of LEL and UEL of studied binary gas mixtures for various industrial applications.
Keywords: explosion parameters, initial temperatures, gas mixture, emissions
Abstrakt
Tato data jsou velmi zajímavá, protože předchozí studie jsou publikovány pouze pro čisté plyny. Získaná data mohou být velice užitečná pro stanovení bezpečnostních opatření zařízení a technologií s různými koncentracemi čistých plynů, směsí s různými stechiometrickými poměry a dokonce s různými počátečními teplotami (také velmi důležitými v průmyslu). Pro budoucí studie navrhujeme stanovení LEL a UEL binárních směsí plynů pro různé průmyslové aplikace.
Klíčová slova: výbuchové parametry, různé počáteční teploty, směs plynů, emise
Přijat k publikování / Received for publication 22. 11. 2019
1. Introduction
In the industrial field, explosions are unfortunately quite common phenomena and have already killed too much people. We must know that the proportion of human losses due to explosions is about 75 % of total losses, only 20 % for the area of fires, and the rest belongs to the toxic release [1]. Previous studies shown the explosion parameters of both separated components (methane/air and hydrogen/air), and there is some correlations for the mixture methane/hydrogen. Explosion characteristics of hydrogen-nitrogen-air mixtures at elevated pressures and temperatures [2]. Experimental and numerical analysis of laminar burning velocity of binary and ternary Hydrocarbon/Hydrogen mixtures [3]. Comparison of explosion characteristics between Hydrogen/air and Methane/air at the stoichiometric concentrations [4].
The explosion characteristics of methane, hydrogen and their mixtures: A computational study [5].
2. Experiment
Explosion characteristic values of the were determined experimentally according to the EN 15967:2012 [6]. The 0.02 m3 (OZM Research, s.r.o.) oil-heated spherical vessel setup was used to record pressure–time curves. The test apparatus consisted of a test vessel, an external heating system, a setup for special atmosphere development (vacuum pump, dispersion nozzle, and high-pressure air), pressure and flash measuring systems, a discharge type ignition system, a temperature measuring device, a user interface, and a data storage system. The test vessel was designed to withstand a working pressure of 30 bar. For testing, the explosion vessel was heated to the desired temperatures of 298-353. The 0.02 m3 apparatus incorporated a digitally adjustable external control device Presto A30 (SN: 10291377, JULABO GmbH, Seelbach, Germany) that heated the oil in the instrument to the specified temperature range. The initial temperature of mixtures was measured with an accuracy of within 1 K. The test mixture was prepared by a partial pressure method with maximum sampling uncertainty ±0.2%. The dynamic pressure in the vessel was measured by a pair of quartz pressure sensors (SN: 4512821 and SN: 4512822, model 701A, Kistler, Winterthur, Switzerland) and recorded with a charge amplifier (Kistler, model 5041E0). The pressure measuring range for the sensors was within 25 bar. The data acquisition sampling period was 0.02 ms with a sampling frequency 50 kS/s. Programmable logic controller (model 5073A211, Siemens, Munich, Germany) connected to a PC was used with the interface (PROMOTIC system, MICROSYS, spol. s.r.o., Ostrava, Czech Republic) to automatically control the whole testing procedure. The experimental setup is schematically introduced in Figure 1.

Figure 1: Experimental setup of the 0.02 m3 spherical vessel
3. Data analysis
The pex was determined as the highest value of the pressure–time curve divided by the initial pressure for the actual equivalence ratio. The pmax is the highest explosion pressure for all equivalence ratios divided by the initial pressure. The value of (dp/dt)max decreases with increasing vessel volume. Therefore, (dp/dt)max is for 0.02 m3 recalculated to a vessel volume (V) of 1 m3. The maximum explosion pressures, the deflagration indexes, and the explosion delay times were obtained from pressure time records. The explosion delay time, tex, is the time at which the maximum explosion pressure is reached. The experimental uncertainty in a statistical sense is given by the used experimental method and is 10% for explosion characteristics according to EN 15967:2011 [6]. An illustration of the smoothing filter on the pressure–time curve determination of the explosion pressure rise is in figure 2.
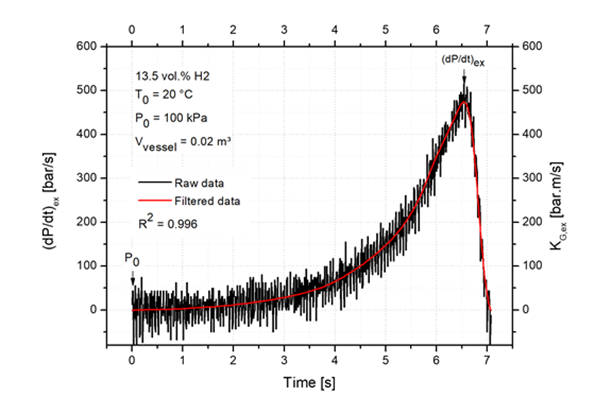
Figure 2: An illustration of the smoothing filter on the pressure–time curve determination of the explosion pressure rise
4. Results and discussions
Method for calculating equilibrium compositions at a specified temperature have been used. The method is based on the minimisation of free energy (NASA method). The maximum possible concentration of each species, based on the availability of each element it contains, is identified and the estimate passed to each solution iteration is not allowed to exceed this value, or fall below zero. This is intended to prevent the solution entering unrealistic regions where it is less likely to converge. Reactants with zero concentration and products containing elements with zero concentration in the reactants are not passed to the DLL. The temperature of the products is adjusted until the equilibrium composition has an enthalpy (or internal energy under constant volume conditions) which is the same as that of the reactants. A starting estimate is improved using the secant method [7].
The results of fuel–air mixtures were used to measure the initial values for pure H2-CH4-air measurements, as presented in Figure 3-4.
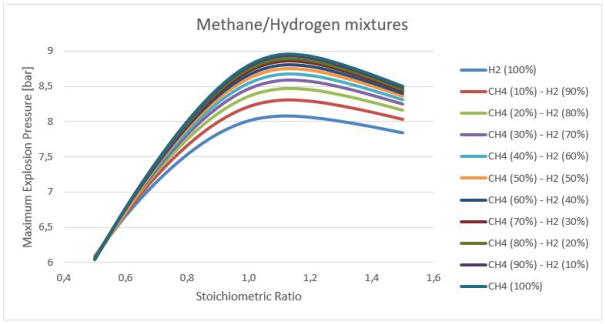
Figure 3: pad/time for H2-CH4-air at p0 = 100 kPa and T0 = 293 K
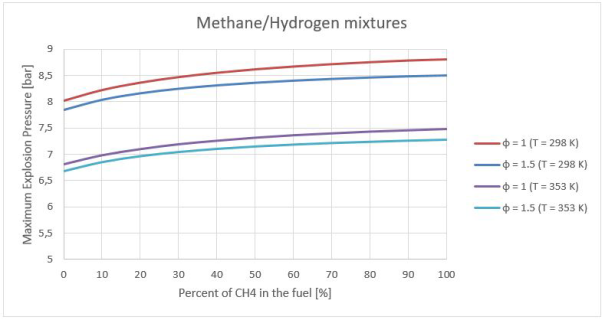
Figure 4: pad/time for H2-CH4-air at p0 = 100 kPa and T0 = 298 K and 353 K
Figures 3-4 illustrates the calculated effects of initial concentration and temperature on the explosion pressure, at the initial temperatures of 298 and 353 K, atmospheric pressure, a p0 of 100 kPa. With increasing temperature, the explosion pressure decreased. The explosion pressure changes approximately 2.0 bar in the maximum values for both temperatures. This value is far behind the experimental error 10%.
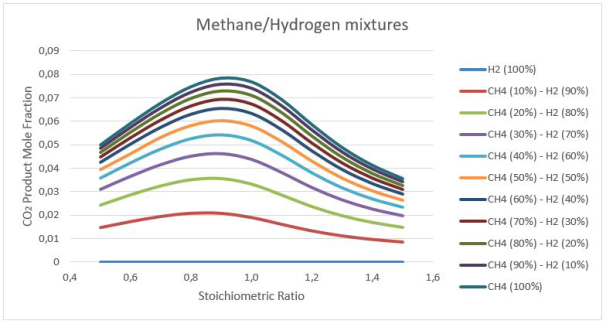
Figure 5: Carbon dioxide products calculations at stoichiometry 0.5-1.5
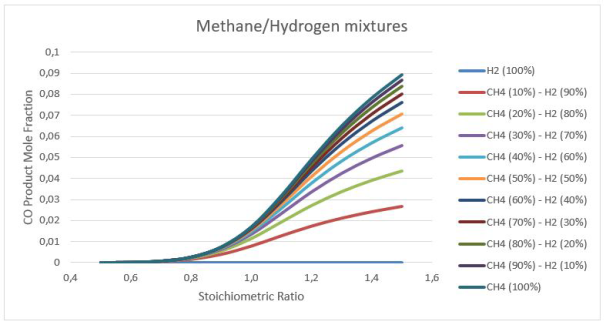
Figure 6: Carbon monoxide products calculations at stoichiometry 0.5-1.5
Figures 5-6 illustrates the effects of initial mixture composition on the production of explosion products. We can easily see that adding Hydrogen to Methane decreases significantly the level of CO2 produced from maximum 7.8 % to 2 % for the 10 - 90 Methane/Hydrogen mixture. Increasing the amount of fuel in the mixture (go to rich mixtures) can be also a way to reduce the CO2, but we can see on the figure 6 that it's not a good solution.Indeed, if we use rich mixtures, the CO2 decreases but the CO increases with the same scale. The CO2 is a greenhouse gas bad for the environment but CO is worst because it's bad for the health.
5. Conclusions
In this work, the experimental study of the explosion characteristics of H2-CH4-air mixtures at different equivalence ratios and initial temperatures was performed. H2-CH4-air mixtures have been characterized in the gas phase by 0.02 m3 for the first time. The presented data allowed to compare the explosion characteristics of the binary mixtures compounds at low initial temperatures. An extensive set of explosion characteristics was obtained, allowing a systematic comparison of the explosion behavior properties to be made among the entire series.
6. Acknowledgement
This work would not have been possible without the financial support of the grant Innovation for Efficiency and Environment - Growth, reg. no. LO1403 supported by National Programme for Sustainability and financed by the Ministry of Education, Youth and Sports.and specific research SP 2019/89.
7. Literature
[1] Investigation [online]. CSB: Chemical safety board [cit. 2019-12-20]. Dostupný z: http://www.csb.gov/investigations/.
[2] TANG, Chenglong; HUANG, Zuohua; JIN, Chun. International Journal of Hydrogen Energy. 2009, vol. 34, pp. 554-561.
[3] BASCO, A.; CAMMAROTA, F.; DI BENEDETTO, A. Chemical Engineering Transactions. 2012, vol. 26, pp. 381-386.
[4] LI, Dong ...[et al.]. International Journal of Hydrogen Energy. 2015, vol. 40, pp. 8761-8768.
[5] MAHDI, F.; XIAOLONG, G.; ZHENG, C. Journal of Loss Prevention in the Process Industries. 2016, vol. 40, pp. 131-138.
[6] EN 15967. Determination of Maximum Explosion Pressure and the Maximum Rate of Pressure Rise of Gases and Vapours. Brussels: European Committee for Standardization, 2011.
[7] Gaseq: Chemical Equilibrium program for Windows [online]. 2004 [cit. 2019-12-20]. Dostupný z: http://www.gaseq.co.uk/.
Vzorová citace
DAUNE, Kevin; SKŘÍNSKÝ, Jan. Effects of Hydrogen Addition on Explosion Characteristics of Methan-air Mixture. Časopis výzkumu a aplikací v profesionální bezpečnosti [online]. 2019, roč. 12, č. 3-4. Dostupný z: https://www.bozpinfo.cz/josra/effects-hydrogen-addition-explosion-characteristics-methan-air-mixture. ISSN 1803-3687.
Užitečné odkazy
Provozovatel portálu
Jeruzalémská 1283/9
110 00 Praha 1


