Release of explosive substances in the process industry due to material corrosion
Únik výbušných látek v procesním průmyslu následkem koroze materiálu
Jan Skřínský
Výzkumné energetické centrum, VŠB-TU Ostrava, 17. Listopadu 15/2172, 708 33 Ostrava, Česká republika; tel. +420 597 324 931, e-mail: jan.skrinsky@vsb.cz
Abstract
Process industry continues to be of central importance to the global economy. At the same time refineries are also large, complex sites with many processes, several of which operate at very high levels of pressure and temperature, and a vast pipeline to transport process fluids throughout the site and eventually to external modes of transport. This combination of factors make refinery sites very vulnerable to a variety of corrosion phenomena that can eventually cause a loss of containment of process fluids, sometimes leading to a serious accident affecting workers, the environment, the surrounding economy and even on occasion the larger economy. This study of corrosion-related accident in refinery is based on important refinery accident in which corrosion of an equipment was identified or suspected as being the key failure leading to the accident event. In this paper, lower explosion limit, LEL, upper explosion limit, UEL, and maximum explosion pressure of coke oven gas measured in 20 dm3 explosion autoclave at 20 °C and 101 kPa, are presented in this paper. Furthermore, the presented measured values at 1.0 bar, 0.75 bar and 0.5 bar and temperatures 323 K and 373 K.
Keywords: mixture, 20 dm3 autoclave, explosive gas, lower explosion limit, maximum explosion pressure, maximum rate of pressure rise, deflagration index release, ALOHA, Google Earth Pro, free-ware program
Abstrakt
Procesní průmysl má stále zásadní význam pro globální ekonomiku. Současně rafinérie představují rozsáhlé, složité podniky s mnoha procesy, z nichž některé pracují při velmi vysokých tlacích a teplotách a využívají dlouhá potrubí pro dopravu tekutin v místě podniku a případně i do dalších částí mimo podnik. Tato kombinace faktorů činí rafinérské lokality velmi zranitelnými vůči různým jevům koroze, které mohou nakonec způsobit ztrátu kontejnmentu provozních tekutin, což někdy vede k vážné havárii postihující pracovníky, životní prostředí, okolní ekonomiku a dokonce i příležitost větší ekonomiky. Tato studie vychází z havárie způsobené korozí v rafinérii, při nichž byla zjištěna koroze zařízení, nebo se předpokládalo, že je to klíčová příčina vedoucí k havárii. V tomto článku jsou prezentovány dolní mez výbušnosti, LEL, horní mez výbušnosti, UEL, a maximální výbuchový tlak koksárenského plynu, změřené v 20 dm3výbuchovém autoklávu při teplotě 20 °C a tlaku 101 kPa. Dále jsou prezentovány změřené hodnoty při tlacích 1.0 bar, 0.75 bar a 0.5 bar a teplotách 323 K a 373 K.
Klíčová slova: směs, 20 dm3 autokláv, výbušný plyn, meze výbušnosti, maximální výbuchový tlak, ALOHA, Google Earth Pro, volně stažitelný program
1. Introduction
In particular, the economic impact of corrosion phenomena and its consequences on refineries is significant, taking into account maintenance and repair costs and production loss from planned and unplanned shutdowns. In the past, many accidents with dangerous substances have occurred worldwide. The most frequent accidents were releases (67%), some of which were followed by explosions (30%) [1]. After evaluating historical accidents and related statistical sources, the following hazardous substance hydrogen as flammable corrosive gas was finally chosen as representative for experiment and simulation. Coke oven gas-air (COG-air) is widely used mixture to demonstrate the validity of any new theoretical or experimental approach to determine its combustion or explosion parameters due to availability of many theoretical and experimental results using various techniques [2,3]. COG as a very complex mixture has high industrial application range. In this case, study two atmospheric issues were highlighted in two separate cases: D (the most frequent conditions during the year → the most probable scenario) and F (the worst dispersion conditions, cloud impact of the largest area → the worst-case scenario).
2. Experimental set-up
The experimental arrangement in Fig. 2 consists of a 20-dm3 spherical stainless steel vessel (OZM Research, s.r.o.) with working pressure up to 30 bar, a dosing vessel, gauge devices for liquid vapor and air and a heating chamber. The explosion vessel was heated up to the required temperatures. The vessel was evacuated to a pressure 40 mbar, filled with the fuel, filled up to 1 bar and purged by 3 times its volume by air. Before ignition, the mixture was allowed to become quiescent and thermally equilibrated. Care was taken not to warm up the equipment by explosions by temperature control unit Presto A30, (JULABO GmbH). The fuel mixtures were prepared by mixing together air and fuel by inner stirrer. By the manufacturer, guaranteed mass fraction purity of COG mixture used in the present study is more than 99.9 %.
![Spherical vessel experimental arrangement [2]](/sites/default/files/obsah/josra/release-explosive-substances-process-industry-due-material-corrosion/obrazky/release-explosives-corrosion01.jpg)
Figure 1: Spherical vessel experimental arrangement [2]
3. Results
3.1 Results of experiment
Fig. 2 illustrates the normalized peak explosion pressure (Pmax/P0) versus equivalence ratio at atmospheric pressure and four initial temperatures. The maximum explosion pressure, pmax, was determined as the highest pex found for the mixture compositions investigated (Fig. 2). The peak explosion pressure (Pmax/P0) is the maximum value of explosion pressure, as indicated in Fig. 2. With the increase of temperature, the peak explosion pressure is decreased due to the decrease of the total fuel mass. Besides, the peak explosion pressure is decreased. This is because the species in flames at elevated temperatures are more reactive, resulting in the promotion of the whole reactions and hence the faster flame speed. Specifically, the laminar flame speed of fuel-air mixture increases with the initial temperature increasing from 323 to 373 K at the equivalence ratio of 1.0.
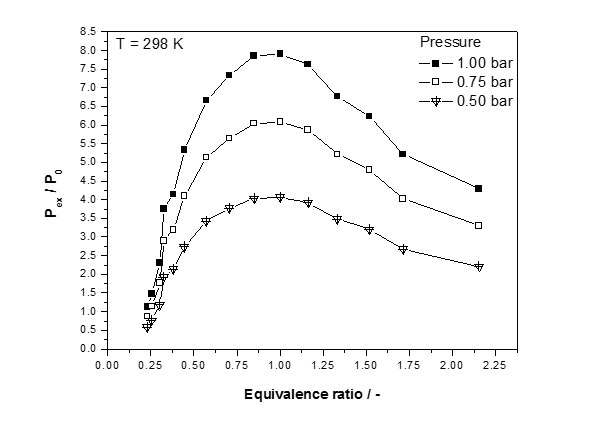
Figure 2: Normalized explosion pressure, Pex/P0, versus equivalence ratios (Ф=0.25-1.00-2.16) at ambient initial temperature, T0=298 K and three different initial pressures for COG
As observed from the experimental results, explosion pressure shows a certain regularity with the variation of coke oven gas concentration (as shown in Fig. 2), and the maximum pressure achieves its peak value near the stoichiometric concentration (equivalence ratio = 1.00) and tends to decrease if the concentration is lower or higher than 1.00. This pattern forms because near the stoichiometric concentration, the coke oven gas and oxygen can be fully utilized, causing the most intense reaction to occur, and thus, the largest pressure value is generated. Investigated mixture display a marked increase in the value of the upper limit as the initial mixture pressure is decrease bellow atmospheric. Concurrently, the lower limit is not changing. Reduction of initial pressure below atmospheric narrows the range where no mixture of a particular fuel-oxidant combination is flammable.
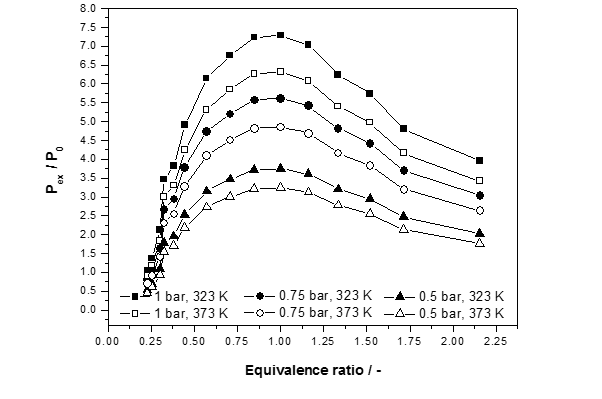
Figure 3: Normalized explosion pressure, Pex/P0, versus equivalence ratios (Ф=0.25-2.16) at different initial temperatures and three different initial pressures for COG
Fig. 3 illustrates the normalized explosion pressure, Pex/P0, versus equivalence ratios. When the concentration of coke oven gas is less than the stoichiometric concentration, even though there is surplus oxygen, the coke oven gas is also in relatively shorter supply, so the explosion is relatively weaker, correspondingly producing relatively less pressure. When concentration of coke oven gas is more than the stoichiometric concentration, the oxygen concentration will relatively low. Thus, the coke oven gas concentration actually involved in the reaction is lower, so less pressure is produced. The greater the concentration of coke oven gas, the less oxygen content, and therefore the less of the coke oven gas actually participates in the reaction. Thus, the pressure becomes smaller. The explosion limits have widen with an increase in initial temperature.
An experimental study on the explosion characteristics of COG/air mixtures was conducted with mathematical modelling. The following Tab. 1 summarized the experimental results for atmospheric conditions.
| Characteristic | Value |
| COG (Ф) | 1.0 |
| pmax (bar) | 8.2±0.16 |
| (dp/dt)max (bar/s) | 180±18 |
| KG (bar·m/s) | 133±49 |
| LEL (obj. %) | 5.6-0.2 |
| UEL (obj. %) | 35.2+0.2 |
Table 1: Explosion parameters of COG at atmospheric conditions
3.2 Results of applications
For the details about a real or potential corrosive gas release and the threat zone the Areal Locations of Hazardous Atmospheres (ALOHA) [4] was used. ALOHA is an atmospheric dispersion model used for evaluating releases of hazardous chemical vapors. ALOHA allows the user to estimate the downwind dispersion of a chemical cloud based on the toxicological/physical characteristics of the released chemical, atmospheric conditions, and specific circumstances of the release. ALOHA can estimate threat zones associated with several types of hazardous chemical releases, including toxic gas clouds, fires, and explosions. Threat zones can be displayed on Google Earth Pro [5] maps to help users assess geospatial information, such as whether vulnerable locations (such as hospitals and schools) might be impacted by the release or whether other nearby factors (such as construction zones) might complicate the response. Two main releases of the selected substances were modelled: instantaneous release (in duration less than 60 s) as the worst-case scenario (catastrophic rupture – 300 mm hole), and semi-continuous release as an alternative scenario representing a more probable accidental situation (50 mm hole). The CO gas was used as a toxic chemical for mathematical modelling.
The most important meteorological conditions influencing the dispersion of corrosive substances are direction and speed of wind, stability class and air temperature. Regarding many possibilities in real situations, the following basic conditions were finally chosen for modelling: The neutral stability of atmosphere [D class; medium wind speed of 5ms-1 (the most frequent conditions during the year → the most probable scenario)]. The very stable stability of atmosphere [F class; low-wind speed of 1.7 m.s-1 (the worst dispersion conditions, cloud impact of the largest area - the worst-case scenario)] [6]. The results of modelling are shown in Fig. 4-5.
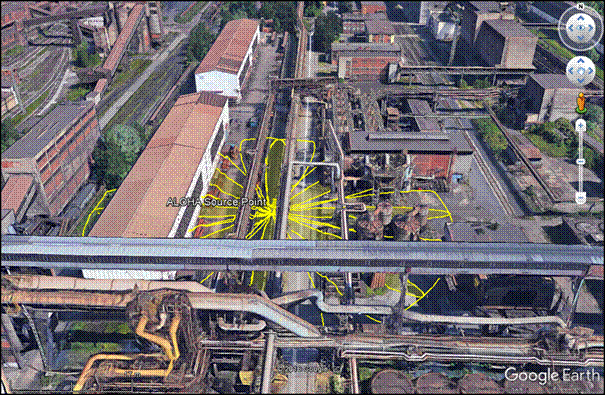
Figure 4: Instantaneous release of corrosive gas from 300 mm hole at stability D
Fig. 4 shows as an airborne chemical travels downwind, it mixes with air. A cloud containing a flammable chemical within its flammability limits can ignite if it encounters a spark, flame, or other ignition source. The vapor cloud combustions are deflagrations that propagate slowly and do not produce blast waves; these are usually referred to as flash fires. For some highly reactive chemicals, the flame speed (the propagation speed) within part of the cloud is accelerated by turbulence caused by obstacles or confinement resulting in a fast deflagration or transition to detonation; either is referred to as an explosion. Both Fig. 4-5 depicted the fatal zones for investigated substance represent 60% of LEL determined experimentally for a potential flash fire or vapor cloud explosion. These events can generate blast waves; usually only a small part of the flammable cloud is involved, so the blast effects are limited. In rare events, a high-power triggering event such as confined vapor cloud explosion can set off the explosion of the entire flammable cloud.
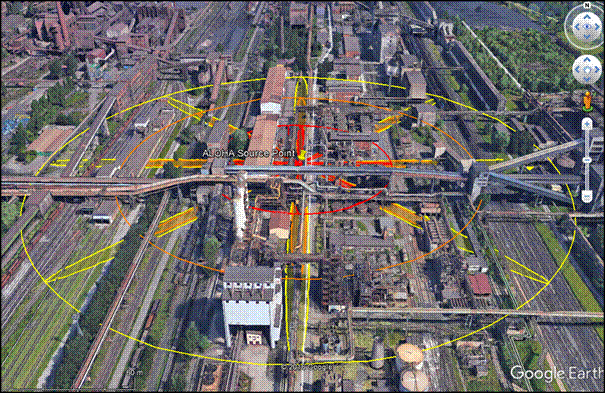
Figure 5: Semi-continuous release of corrosive gas from 50 mm hole at stability F
The overall results show a high-level severity in cases of both corrosion related accidents within industrial zones. Taking into account the selected dangerous substance, the second scenario in Fig. 5 could cause the largest fatal zone. When considering road or rail transport accidents, the radius of affected zone is up about 200 m. A shorter radius of the order of tens of metres characterizes the results of instantaneous release dispersion modelling. It should be noted that standard software either neglects humidity and topography, or is relatively insensitive to variations in them. Therefore, CFD modelling should explore these conditions. Of all potential impacts, the financial impact of explosive corrosion gas release in this case will be the most consistently and alarmingly high.
4. Conclusion
Corrosion-related accident in petroleum refinery was simulated with the experimentally obtained LEL explosion parameters as inputs for modelling.
The main conclusions are summarized as follows:
- The explosion pressures of coke oven gas-air mixtures attained their highest value at a fuel-air ratio 1 within the studied concentration ranges.
- The explosion pressure, the maximum rate of pressure rise, the deflagration index and the combustion duration are decreased, while the normalized mass burning rate is increased with the increase of initial temperature.
- 0.25 and 2.15 fuel-air mixture presents the shortest and the longest combustion duration and flame development period, indicating the fastest and slowest flame speed.
- The increase of initial temperature linearly increase the LEL.
- The severity of catastrophic and of semi-continuous scenarios; distances of hazardous zones under two types of weather conditions (the most frequent versus the worst condition from the point of view of cloud dispersion) have been determined.
The results represents a continuation of numerous efforts by various research groups, where the key underlying problem has been the understanding of results obtained in laboratory tests for predicting the consequences of gas/vapor explosion scenarios in industry [7-8]. Our results can be employed when preparing more sophisticated simulations based on computational fluid dynamics modelling, and aimed at investigation of the limitations of standard models on one hand, and their improvement on the other hand.
Acknowledgement
This work would not have been possible without the financial support of Innovation for Efficiency and Environment - Growth, reg. no. LO1403 supported by National Programme for Sustainability and financed by the Ministry of Education, Youth and Sports.
References
[1] LEES, F. P. Loss Prevention in the Process Industries: Hazard Identification, Assessment, and Controls: vol. 1-3. 4th ed. Oxford: Butterworth-Heinemann, 2012.
[2] OVILEANU, C. ...[et al.]. Combustion Processes. Rev. Chim. 2011, Vol. 62, pp. 376-379.
[3] PEKALSKI, A. ...[et al.]. Process Saf. Environ. 2005, Vol. 83, pp. 421-429.
[4] ALOHA® 5.4.7: Areal Locations of Hazardous Atmospheres [online]. U.S. Environmental Protection Egency and National Oceanic and Atmospheric Administration, 2017 [cit. 2017-02-14]. Dostupný z: https://www.epa.gov/cameo/aloha-software.
[5] Google Earth Pro® 7.1.7.2606: multiplatformní virtuální globus [online]. Google, 2017 [cit. 2017-02-14]. Dostupný z: https://www.google.com/earth/download/gep/agree.html.
[6] BERNATIK, A. ...[et al.]. Process Safety and Environmental Protection. 2008, Vol. 86, No.3, pp. 198-207.
[7] SKŘÍNSKÁ, M. ...[et al.]. WSEAS Transactions on Heat and Mass Transfer. Vol. 9, No. 1, pp. 243-250.
[8] SKŘÍNSKÝ, J. Materials Science Forum. 2016, Vol. 844, pp. 65-72.
Vzorová citace
SKŘÍNSKÝ, Jan. Release os explosive substances in the proces industry due to material corrosion. Časopis výzkumu a aplikací v profesionální bezpečnosti [online]. 2017, roč. 10, č. 3-4. Dostupný z: http://www.bozpinfo.cz/josra/release-explosive-substances-process-industry-due-material-corrosion. ISSN 1803-3687.
Užitečné odkazy
Provozovatel portálu
Jeruzalémská 1283/9
110 00 Praha 1


