Maximum explosion pressure of H2/O2/N2 mixture at various initial pressures
Maximální výbuchový tlak směsi H2/O2/N2 pro různé počáteční tlaky
Jan SKŘÍNSKÝ1, Ján VEREŠ1, Jana TRÁVNÍČKOVÁ1, Andrea DALECKÁ1
1Energy Research Centre, VSB-Technical University of Ostrava, corresponding author: jan.skrinsky@vsb.cz
Abstract
A theoretical study on maximum explosion pressure is presented. The maximum explosion pressures, computed by assuming chemical equilibrium within the explosion front are examined in comparison with the measured explosion pressures. Comparisons of the experimentally measured pressures with the calculated adiabatic pressures indicate the degree of adiabacity of the explosion. The calculated peak explosion pressures of hydrogen-air mixtures for ambient conditions are examined in comparison with the experimental values and with the calculated adiabatic explosion pressures. In the present contribution we calculated the maximum pressure for hydrogen-air mixtures in a spherical closed volume at different initial temperatures up to 200 °C. The results represents a continuation of numerous efforts by various research groups, where the key underlying problem has been the understanding of results obtained in laboratory tests for predicting the consequences of gas explosion scenarios in industry.
Keywords: Maximum Explosion Pressure; Elevated Pressure; Hydrogen
Abstrakt
V článku je prezentována teoretická studie stanovení maximálního výbuchového tlaku. Maximální výbuchový tlak je vypočítán za předpokladu vzniku chemické rovnováhy na čele výbuchové vlny a srovnán s naměřenými výbuchovými tlaky. Porovnání experimentálně zaměřených tlaků s vypočtenými adiabatických tlaky demonstruje stupeň adiabacity exploze. Vypočítaný maximální výbuchový tlak pro různé směsi vodíku se vzduchem pro standardní podmínky je srovnán s experimentálními hodnotami a s vypočtenými adiabatickým výbuchovým tlakem. V tomto příspěvku je vypočítán maximální výbuchový tlak pro směsi vodíku se vzduchem pro případ kulového uzavřeného objemu pro různé počáteční teploty až do hodnoty 200 °C. Výsledky představují pokračování v úsilí různých výzkumných skupin v oblasti porozumění datům získaných v laboratorních testech pro predikci následků scénářů výbuchů plynů v průmyslu.
Klíčová slova: Maximální výbuchový tlak; Zvýšený počáteční tlak; Vodík
1. Introduction
Hydrogen-air is together with methane-air the most widely used mixture to demonstrate the validity of any new theoretical or experimental approach to determine its combustion or explosion parameters due to availability of many theoretical and experimental results using various techniques (Pekalski, 2005; Movileanu et al., 2011). The characteristic parameter of hydrogen in a closed vessel explosion, so called explosion characteristic, discussed in this contribution is the maximum explosion pressure. The maximum explosion pressure is the highest explosion pressure over the flammable range in a closed volume at a given fuel concentration (Movileanu et al., 2011). According to standards, the maximum explosion pressure is determined experimentally by preparing test mixtures of hydrogen and air as oxidizing gas and conducting ignition tests at ambient conditions. Experiments could be, however, expensive and time consuming, especially at elevated conditions of temperature and pressure, at which many industrial processes occur (Pekalski, 2005). Therefore, approximate calculation methods are being sought with which approximate values of explosion pressure can be calculated in reasonable time. In the field of explosion prevention and for the proper use of this safety characteristic it is necessary to know its transferability to practical non ambient conditions. The present contribution presents explosion pressures of stoichiometric hydrogen - air mixtures calculated for various initial temperatures. The temperature influence on the maximum explosion pressure was investigated at atmospheric pressure.
2. Previous studies
Results of explosion experiments depend on many different parameters of the investigated process, such as the energy and type of ignition source, size and shape of explosion chamber, initial temperature, initial pressure and composition of the flammable mixture. To ensure the compatibility of data we selected the results for experiments that are in agreement with EN 13673-1 with the only exception that is volume of the testing vessel. (Holtappels et al., 2002) reports measurement of explosion pressures of hydrogen-air mixtures, at various initial concentrations close to stoichiometric (9.5-10.5 vol.%), in closed vessels of different volumes. The results have shown a similar behavior of investigated system. The maximum explosion pressures are (7.9±0.3) bar(a) for measurements made in the standard 20.0×10-3 m3 spherical vessel. Higher deviations from these values are observed, however, when using smaller or larger size vessels, where radiative and convective heat losses to the walls could be neglected. (Cashdollar et al., 2000) reports different value of maximum explosion pressures for hydrogen-air mixtures, depending on the volume of the explosion vessel: 8.1 bar(a) in a 120.0×10-3 m3 explosion vessel. The reported results from 6.0×10-3 are identical to those results obtained in the 20.0×10-3 m3 and 120.0×10-3 m3 despite the great difference in volume. There are not reported experimental studies for 1 m3.
3. Analysis
Mathematical model used in this study computes adiabatic flame temperatures and constant volume adiabatic explosion pressures at various initial temperatures and pressures, taking into account 26 species. It has been shown (Skrinsky et al., 2015) that the model is able to predict, with a reasonable accuracy in different fuel-enriched conditions, for different types of gaseous mixtures explosions.
Element balanced equation:
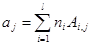 (1)
(1)
where ajare moles of j-th element (atom); niare moles of i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; j is the number of species.
The free energy (Helmholtz function) of the system F equation:
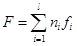 (2)
(2)
where F is the free energy (Helmholtz function) of the system; ni are moles of i-th species; fi is free energy of i-th species.
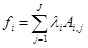 (3)
(3)
where fi is free energy of i-th species; λi are the Lagrangian multipliers for each element (j=1 to J); Ai,jnumber of j-th element in i-th species.
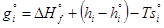 (4)
(4)
where gi is the Gibbs free energy for single i-th species; ΔHfis the enthalpy of formation of the i-th species at standard conditions;
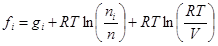 (5)
(5)
where giis the Gibbs free energy for single i-th species; R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
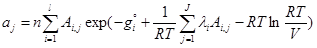 (6)
(6)
where ajare moles of j-th element (atom); niare moles of i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; j is the number of species; gi is the Gibbs free energy for single i-th species; λi are the Lagrangian multipliers for each element (j=1 to J); R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
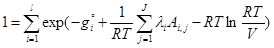 (7)
(7)
where gi is the Gibbs free energy for single i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; λi are the Lagrangian multipliers for each element (j=1 to J); R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
The equations 1-7 were used to investigate and quantify the role of initial temperature and pressure in affecting the explosion behaviour of H2/O2/N2 mixtures.
4. Results and discussions
A) Adiabatic temperatures and explosion pressures for H2/O2/N2
Calculated adiabatic temperatures, Tad, and explosion pressures, Pex, for H2/O2/N2 were determined at various initial temperatures and ambient initial pressure. The results of calculations are given in Table 1.
|
Fuel fraction [vol.%] |
Initial temperature (K) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 298 | 358 | 418 | 478 | |||||
| Pex | Tad | Pex | Tad | Pex | Tad | Pex | Tad | |
| 4.50 | 2.62 | 798 | 2.33 | 852 | 2.12 | 905 | 1.96 | 958 |
| 27.5 | 7.84 | 2679 | 6.58 | 2696 | 5.68 | 2712 | 5.01 | 2729 |
| 50.5 | 4.14 | 2334 | 5.95 | 2372 | 5.17 | 2408 | 4.59 | 2444 |
| 75.0 | 4.60 | 1426 | 3.96 | 1477 | 3.51 | 1527 | 3.17 | 1578 |
Table 1: Computed explosion pressures for H2/O2/N2 mixtures at p0 = 1 bar(a)
From the numerical results of Table 1 it is possible to identify that the increase in the initial temperature lowers the maximum explosion pressure, and increases the flammability range. The value of the explosion pressure with varying H2/O2/N2 concentration is similar at all investigated initial temperatures. The maximum value of the explosion pressure is found close to 30 vol. % of H2 for all conditions. Further, in Table 2, we reported simulations on the explosion properties of H2/O2/N2 mixtures at various initial pressure and ambient initial temperature.
|
Fuel fraction [vol.%] |
Initial pressure (bar(a)) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | |||||
| Pex | Tad | Pex | Tad | Pex | Tad | Pex | Tad | |
| 4.50 | 2.62 | 798 | 13.10 | 798 | 26.19 | 798 | 39.29 | 798 |
| 27.5 | 7.84 | 2679 | 39.94 | 2740 | 80.36 | 2761 | 120.91 | 2771 |
| 50.5 | 7.03 | 2334 | 35.28 | 2345 | 70.62 | 2348 | 105.97 | 2349 |
| 75.0 | 4.54 | 1426 | 22.70 | 1427 | 45.40 | 1428 | 68.10 | 1428 |
Table 2: Computed explosion pressures for H2/O2/N2 mixtures at T0 = 298 K
From the data performed in Table 2, it is clear that the increase of initial pressure has significant effect on explosion pressure when only the air as oxidant. This is a good approximation at low temperatures i.e. up to 500 K, but we may assume that will be increasing wrong at higher temperatures where not only the chemistry faster is, but the fraction of radicals is much higher.
a)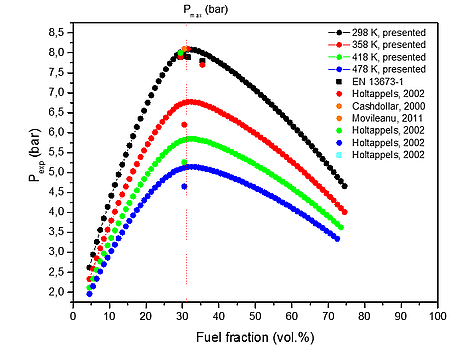
b)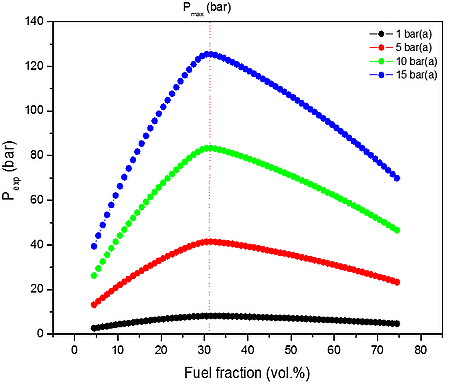
Figure 1: Calculated explosion pressure vs fuel fraction for explosions of a) H2/O2/N2 mixture at 298 K (top), 358 K (upper middle), 418 K (lower middle), and 478 K (bottom); b) H2/O2/N2 mixture at 1 bar(a) (top), 5 bar(a) (upper middle),10 bar(a) (lower middle), and 15 bar(a) (bottom)
a)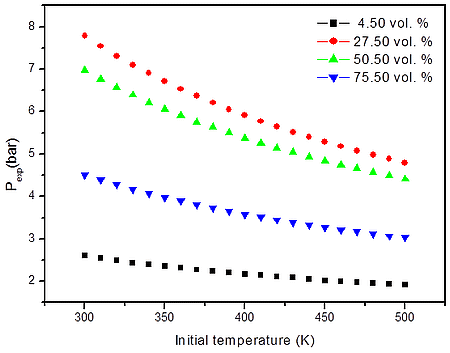
b)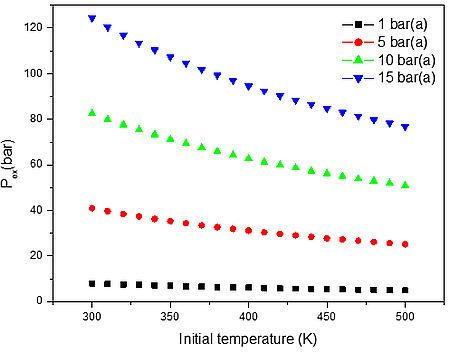
Figure 2: Calculated explosion pressure vs initial temperature for explosions of a) H2/O2/N2 mixture at 27.50 vol. % (top), 50.50 vol. % (upper middle), 75.50 vol. % (lower middle), and 4.50 vol. % (bottom); b) H2/O2/N2 mixture at 1 bar(a) (top), 5 bar(a) (upper middle), 10 bar(a) (lower middle), and 15 bar(a) (bottom)
5. Conclusion
Adiabatic explosion pressures of hydrogen-air mixtures at various initial temperatures were calculated. The influences of initial temperature are compared with the experimental results and with the equilibrium calculations. Although the results from the evaluation indicate that presented theoretical simulations can become a valuable tool for rough estimation, the modeling requires further improvements to be useful for consequence modeling and design of industrial facilities. Thus, at the first stage, the equilibrium calculations can be used as a rough calculation of a worst case scenario. At the same time, these values will be used as approximate initial values for explosion experiments carried out in heated 1 m3 explosion apparatus built by OZM Research s.r.o. at Energy Research Centre, VŠB - Technical University of Ostrava.
6. Acknowledgement
This work would not have been possible without the financial support of two grants: (1) Innovation for Efficiency and Environment, reg. no. ED0036/01/01 supported by Operation Program Research and development for Innovation and financed by the Ministry of Education, Youth and Sports; (2) Innovation for Efficiency and Environment - Growth, reg. no. LO1403 supported by National Programme for Sustainability and financed by the Ministry of Education, Youth and Sports. J.V. is thankful for the financial support of the project “Support research and development in the Moravian-Silesian Region 2014 DT 1-International research teams” 02722/2014/RRC, financed from the budget of the Moravian-Silesian Region. J.S. is thankful to his advisor Ing. Jan Koloničný, Ph.D. and mentor RNDr. Prof. Pavel Danihelka, CSc. for their knowledge support in the area of decision process, industrial explosion safety and enthusiasm for a team work.
7. References
[1] PEKALSKI A. A.; SCHILDBERG, H. P.; SMALLEGANGE P. S. D. …[et al.]. Determination of the Explosion Behaviour of Methane and Propene in Air or Oxygen at Standard and Elevated Conditions. Process Safety and Environmental protection, 2005, vol. 83, B5, pp. 421-429.
[2] MOVILEANU, C.; MITU, M.; BRINZEA, V. …[et al.]. Adiabatic Flame Temperature of Fuel-Air Mixtures in Isobaric and Isochoric Combustion Processes. Rev. Chim., 2011, vol. 62, no. 4, pp. 376-379.
[3] HOCHTAPPELS, K. Report on experimentally determined explosion limits, explosion pressures and rates of explosion pressure rise: part 1: methane, Hydrogen, Propylene. Journal of Loss Prevention in the Process Industries, 2006, vol. 19, n. 4, pp. 334-342.
[4] CASHDOLLAR, K. L. …[et al.]. Flammability of methane, propane, and hydrogen gases. Journal of Loss Prevention in the Process Industries, 2000, vol. 13, no. 3-5, pp. 327–340.
[5] SKŘÍNSKÝ, Jan…[et al.]. Explosion chracteristics of methane at elevated initial temperatures. Časopis výzkumu a aplikací v profesionální bezpečnosti [online], 2015, roč. 8, č. 2-3. Dostupný z WWW: <http://www.bozpinfo.cz/josra/josra-02-03-2015/methane-explosion-characterist.html>. ISSN 1803-3687.
Vzorová citace
SKŘÍNSKÝ, J. …[et al.]. explosion characteristics of syngas mixtures at elevated temperatures and pressures. Časopis výzkumu a aplikací v profesionální bezpečnosti [online], 2015, roč. 8, č. 4. Dostupný z WWW: <http://www.bozpinfo.cz/josra/josra-04-2015/maximum-explosion.html>. ISSN 1803-3687.
Užitečné odkazy
Provozovatel portálu
Jeruzalémská 1283/9
110 00 Praha 1


