Explosion characteristics of methanol-air mixtures at elevated temperatures and pressures - numerical study
VÝBUCHOVÉ CHARAKTERISTIKY směsí metanolu a vzduchu ZA ZVÝŠENÝCH POČÁTEČNÍCH teplot a tlaků - výpočet
Jan Skřínský1, Ján Vereš1
1Energy Research Centre, VSB-Technical University of Ostrava, corresponding author: jan.skrinsky@vsb.cz
Abstract
A numerical study was performed on the explosion characteristics of methanol-air mixtures; at four various initial temperatures and initial pressures. The explosion parameters of explosion pressure were calculated. The influences of initial conditions on the explosion characteristics were discussed. With the initial pressure elevated from 1.0 to 2.5 bar, the peak explosion pressure increases significantly. Post explosion species composition as a function of methanol mole fraction in the methanol-air post-explosion mixtures (15 vol. % of fuel) for P = 1.0 bar(a) and T = 298 K have been determined. These values will be used as approximate initial values for explosion experiments carried out in heated 1 m3 and 20 dm3 explosion apparatuses designed by OZM Research s.r.o. and used at Laboratory of safety fuels and technologies, Energy Research Centre, VŠB - Technical University of Ostrava.
Keywords: Maximum Explosion Pressure; Constant Volume Adiabatic Temperature; Methanol
Abstrakt
Byla provedena numerická studie ke zjištění výbuchových charakteristik směsí metanol - vzduch při čtyřech různých počátečních teplotách a počátečních tlacích. Byly vypočteny parametry adiabatického výbuchového tlaku. Byly diskutovány vlivy počátečních podmínek na výbuchové charakteristiky. S počátečním tlakem od 1,0 do 2,5 bar, dochází k prudkému nárůstu výbuchového tlaku. Bylo stanoveno složení po výbuchové směsi jako funkce molárního podílu metanolu ve směsi pro výbuch směsi metanol - vzduch (15 obj. % paliva) a počáteční podmínky P = 1,0 bar(a) a T = 298 K. Tyto hodnoty budou použity jako přibližné hodnoty pro experimenty v otápěných 1 m3 a 20 dm3 výbuchových komorách navržených OZM Research s.r.o. a dostupných v Laboratoři bezpečnosti paliv a technologií na Výzkumném energetickém centru, VŠB-TU Ostrava.
Klíčová slova: Maximální výbuchový tlak; Adiabatická teplota při konstantním objemu; Metanol
Introduction
Alcohols provide promising potential in improving pollutant emissions and reducing dependency on traditional fossil fuels. Previous research suggests that blending alcohol into traditional fuels benefits the complete combustion in engines and hence the reduction of HC, CO and C(s) soot emissions. Low alcohols, such as methanol, can be mixed with gasoline as octane improver for their high octane number. However, low alcohols are challenging to be used on compression ignition (CI) engine, since additional ignition assistant is necessary [1].
In the utilization of methanol fuel, much work has been concentrated on the combustion and emission characteristics of the engines fueled with pure methanol, alcohols-gasoline and gasoline-alcohols mixtures for high load extension and high fuel efficiency. Engineering-scale production of methanol is being developed in more advanced and cheaper way, motivating related research in all fields. Methanol can be produced from agricultural feed-stocks, forestry wood wastes and agricultural residues. One example for methanol production is rice straw, which is the most abundant lingo-cellulosic biomass worldwide [2].
The characteristic safety parameter of methanol-air mixtures in a closed vessel explosion, so called explosion characteristic, discussed in this contribution is the adiabatic explosion pressure and adiabatic explosion temperature. The maximum explosion pressure is the highest adiabatic explosion pressure over the flammable range in a closed volume at a given fuel concentration [3].
These explosion characteristics are important for design of safety devices (e.g. relief systems, vents), able to ensure active protection of pressure vessels where flammable mixtures are formed. Beyond safety devices, the values of these parameters are useful for emergency planning especially for developing scenarios where emergency relief or external heat transfer may be inadequate. At the same time, the maximum explosion pressure that the explosion reaction can generate is a good measure of the magnitude of the hazard associated with the reaction [4].
Nowadays, experimental tests are carried out in a closed cylindrical vessel. Experiments could be, however, expensive and time consuming, especially at elevated initial temperatures and pressures, at which many industrial processes are operated. Therefore, approximate calculation methods, with which approximate values of explosion pressure can be calculated in reasonable time, are being sought.
Few data at standard conditions are available in open literature. Many processes, especially in the petrochemical industry, however, operate at elevated temperature and pressure. While the values of such explosion parameters at these elevated conditions are essential to safety and reliable operation, they are nevertheless largely unavailable in open literature.
The present contribution presents adiabatic explosion pressures (in terms of bar(a)) of stoichiometric CH3OH/O2/N2 mixtures with air calculated for various initial temperatures and pressures. The aim of this contribution is to evaluate the influence of different initial conditions (concentration, pressure and temperature). The primary outcomes are: explosion parameters at ambient conditions and elevated temperatures and pressures; and the computed post explosion gas composition of the major and minor species in terms of mole %.
Analysis
Mathematical model used in this study computes constant volume adiabatic explosion pressures at various initial temperatures and pressures, taking into account 26 species. The equations 1-7 were used to investigate and quantify the role of initial temperature and pressure in affecting the explosion behavior of methanol-air mixtures. The theory of thermodynamics and chemical equilibrium used for the equations 1-7 is described in [5-6].
Element balanced equation:
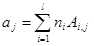 (1)
(1)
where ajare moles of j-th element (atom); niare moles of i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; j is the number of species.
The free energy (Helmholtz function) of the system F equation:
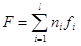 2)
2)
where F is the free energy (Helmholtz function) of the system; ni are moles of i-th species; fi is free energy of i-th species.
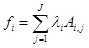 (3)
(3)
where fi is free energy of i-th species; λi are the Lagrangian multipliers for each element (j=1 to J); Ai,jnumber of j-th element in i-th species.
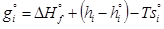 (4)
(4)
where g°i is the Gibbs free energy for single i-th species at standard conditions; ΔH°fis the enthalpy of formation of the i-th species at standard conditions; hi and h°i are specific and standard enthalpies of the i-th species; T is the thermodynamic temperature; s°i is the molar entropy of the i-th species at standard conditions.
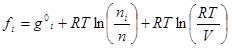 (5)
(5)
where g°iis the Gibbs free energy for single i-th species at standard conditions; R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
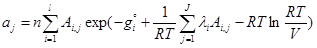 (6)
(6)
where ajare moles of j-th element (atom); niare moles of i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; j is the number of species; gi is the Gibbs free energy for single i-th species at standard conditions; λi are the Lagrangian multipliers for each element (j=1 to J); R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
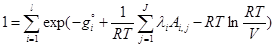 (7)
(7)
where gi is the Gibbs free energy for single i-th species; Ai,jnumber of j-th element in i-th species; l is the number of elements; λi are the Lagrangian multipliers for each element (j=1 to J); R is the universal gas constant; T is temperature; ni is the number of moles of every species; V is the volume.
Results and discussions
Results of explosion characteristics depend on many different parameters of the investigated process, such as initial temperature, initial pressure and composition of the flammable mixture. The results of calculations are summarized in Tables 1-3.
A) Adiabatic Pmaxfor CH3OH/O2/N2 at elevated temperature and pressure
Computed adiabatic explosion pressures, pad, for methanol-air mixtures (5.0 vol. %, 20.0 vol. %, 35.0 vol. % and 50.0 vol. % of fuel) at various initial temperatures (298 K, 358 K, 418 K, 478 K), Tinit, and ambient initial pressure (1 bar), p0, are given in Table 1.
|
Fuel fraction [vol.%] |
Initial temperature (K) |
|||
|---|---|---|---|---|
| 298 | 358 | 418 | 478 | |
| 5.0 | 5.4 | 4.6 | 4.1 | 3.7 |
| 20.0 | 9.1 | 7.7 | 6.8 | 5.9 |
| 35.0 | 6.8 | 5.9 | 5.2 | 4.6 |
| 50.0 | 5.9 | 4.9 | 4.2 | 3.7 |
Table 1: Computed adiabatic explosion pressures for CH3OH/O2/N2 mixtures at p0 = 1 bar(a)
From the numerical results of Table 1, it is possible to identify that the increase in the initial temperature (from 298 K to 478 K) lowers the adiabatic explosion pressure (from 9.07 bar to 5.93 bar for 20.0 vol. % of fuel). The trend of the adiabatic explosion pressure with varying CH3OH/O2/N2 concentration is different at all investigated initial temperatures (e.g. for the concentration 35.0 vol. % of fuel and temperatures 298 K and 358 K it is about 0.9 bar; for temperatures 358 K and 418 K it is about 0.7 bar etc.). The maximum value of the explosion pressure is found close to 15 vol. % of methanol for all temperatures and it is shown in Figure 1. Further, in Table 2, calculations on the adiabatic explosion properties of CH3OH/O2/N2 mixtures at various initial pressures, p, and ambient initial temperature, T0 are reported.
|
Fuel fraction [vol.%] |
Initial pressure (bar(a)) |
|||
|---|---|---|---|---|
| 1 | 1.5 | 2.0 | 2.5 | |
| 5.0 | 5.4 | 8.1 | 10.8 | 13.5 |
| 20.0 | 9.1 | 13.6 | 18.1 | 22.7 |
| 35.0 | 6.8 | 10.3 | 13.7 | 17.1 |
| 50.0 | 5.9 | 8.9 | 12.1 | 15.2 |
Table 2. Computed explosion pressures for CH3OH/O2/N2 mixtures with air at T0 = 298 K
From the data written in Table 2, it is clear that the increase of initial pressure (from 1 bar to 2.5 bar) has significant effect on adiabatic explosion pressure (approximately 2.5 times) when only the oxygen from the air was used as an oxidant. This is an approximation at initial pressures i.e. up to 2.5 bar(a) (with corresponding pad 22.7 bar(a)), because we may assume to use these values as rough conservative predictions for explosion experiments which will be carried out in heated 1 m3 and 20 dm3 explosion apparatuses with working pressures up to 30 bar(a).
a)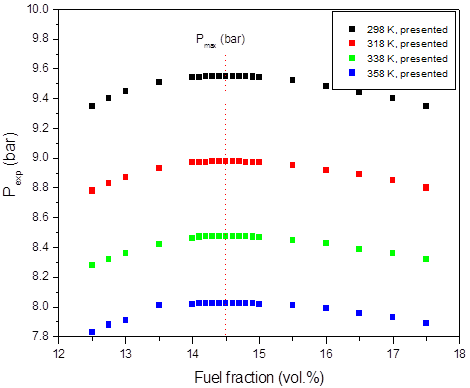
b)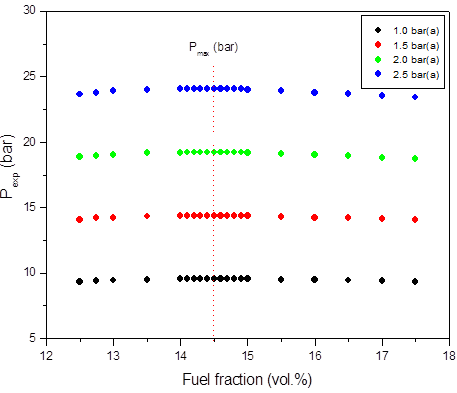
Figure 1: Calculated adiabatic explosion pressure vs fuel fraction for explosions of a) CH3OH/O2/N2 mixtures at 298 K (top), 358 K (upper middle), 418 K (lower middle), and 478 K (bottom); b) CH3OH/O2/N2 mixtures at 1.0 bar(a) (top), 1.5 bar(a) (upper middle), 2.0 bar(a) (lower middle), and 2.5 bar(a) (bottom)
a)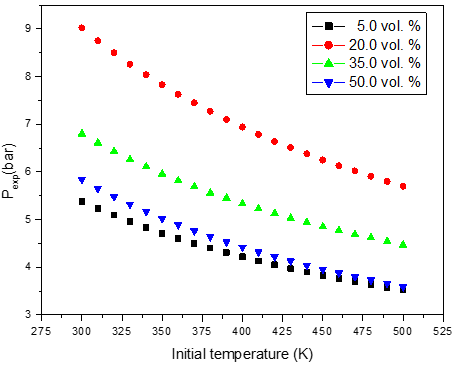
b)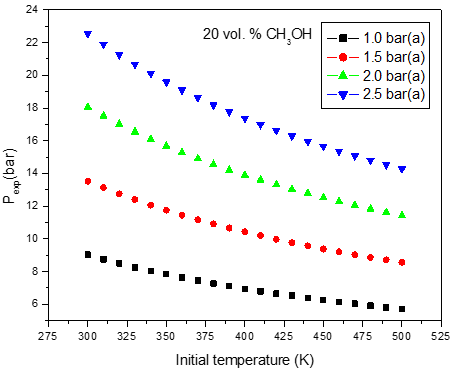
Figure 2: Calculated adiabatic explosion pressure vs initial temperature for explosions of a) CH3OH/O2/N2 mixtures at 5.0 vol. % (top), 20.0 vol. % (upper middle), 35.0 vol. % (lower middle), and 50.0 vol. % (bottom); b) CH3OH/O2/N2 mixtures at 1.0 bar(a) (top), 1.5 bar(a) (upper middle), 2.0 bar(a) (lower middle), and 2.5 bar(a) (bottom)
Figures 1-2 are plots of the calculated explosion pressure vs fuel fraction and calculated explosion pressure vs initial temperature. In Figure 1a, the maximum adiabatic pressures calculated are 9.6 bar(a) (298 K), 8.9 bar(a) (318 K), 8.5 bar(a) (338 K) and 8.0 bar(a) (358 K). In Figure 1b, the maximum adiabatic pressures are 24.1 bar(a) (1.0 bar), 19.2 bar(a) (1.5 bar), 14.4 bar(a) (2.0 bar) and 9.6 bar(a) (2.5 bar). Figures 2a-2b show the calculated explosion pressure behavior of near stoichiometric (20 vol.%), fuel lean (5 vol. %) and fuel rich (50 vol. %) CH3OH/O2/N2 mixtures. The adiabatic pressure trends for mixtures are very similar. Depending on the initial fuel fraction and pressure, they exhibit a single pressure decrease with increasing temperature. The mixture reactivity cannot be quantified through Pex, because increased the initial temperature decreases expansion ratio Tad/Tinit. Dependency of reactivity on initial condition would be seen from results of rate of pressure rise calculations.
B) Post-explosion mixture composition after methanol explosion at ambient and elevated pressures and temperatures
The chemical equilibrium model assumes adiabatic conditions in constant volume, and formation of equilibrium-defined concentrations of post-explosion compounds and their expansion due to the temperature rise caused by the liberated heat assuming ideal gas behavior and ideal condensed phase products. The model assumes homogeneity in the gas, i.e., all molecules in the system participate in the reaction. The equilibrium defined post-explosion gas products for different methanol concentrations (5, 15, 20, 25, 35 and 50 vol. %), p =1.0 bar(a), T = 298 K, are given in Table 3.
| Compound | Species composition in post-explosion mixture (mole %) | |||||
|---|---|---|---|---|---|---|
| 5 | 15 | 20 | 25 | 35 | 50 | |
| N2 | 73.15 | 59.65 | 51.28 | 44.13 | 32.84 | 20.83 |
| O2 | 12.08 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 |
| H2O | 9.75 | 23.64 | 22.40 | 19.51 | 13.20 | 12.77 |
| CO2 | 4.88 | 7.65 | 4.85 | 3.95 | 4.26 | 5.91 |
| CO | 0.00 | 5.69 | 11.38 | 14.67 | 18.12 | 12.89 |
| H2 | 0.00 | 2.81 | 10.02 | 17.73 | 31.56 | 39.96 |
| H | 0.00 | 0.15 | 0.06 | 0.01 | 0.00 | 0.00 |
| NO | 0.13 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 |
| OH | 0.01 | 0.28 | 0.02 | 0.00 | 0.00 | 0.00 |
| NH3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.04 |
| C(S) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 7.60 |
Table 3: Calculated post-explosion gas composition of the main species, p =1.0 bar(a), T = 298 K
In the 15 mole % of methanol and higher concentrations, all of the oxygen is consumed during the explosion. This confirms that concentration of 15 mol % is stoichiometric or near stoichiometric and higher concentrations are on rich side of explosible range. Soot was numerically predicted in the methanol concentrations 50 mole %. As an illustration, the main post explosion species (above 10 mole %) versus the initial methanol concentration, for different initial pressure and temperature are given in Figure 3. The calculated post-explosion composition has not been compared with the experiment. In order to confirm the predictions, the gas chromatography or FTIR analysis will be performed to investigate the experimental composition of post-explosion products.
a)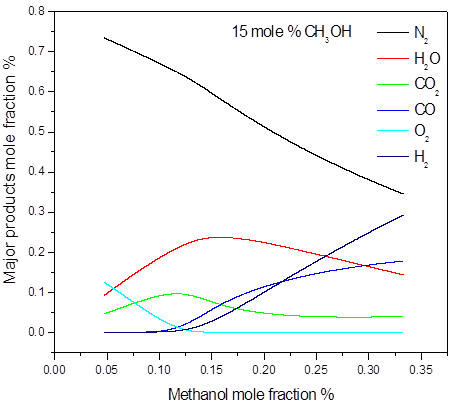
b)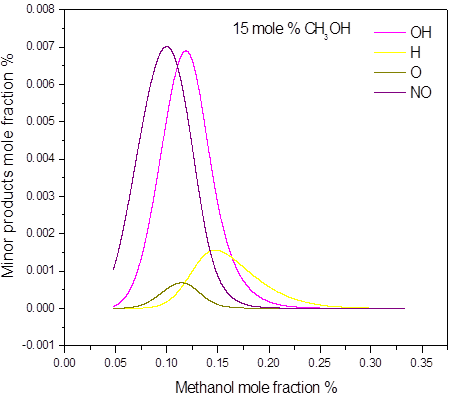
Figure 3. Calculated post-explosion species composition as a function of methanol mole fraction in the methanol-air post-explosion mixtures, P = 1.0 bar(a), T = 298 K: a) major; b) minor products
Conclusions
The adiabatic explosion pressures and temperatures of methanol-air mixtures were comparatively calculated at four elevated initial temperatures (298 K, 358 K, 418 K, 478 K) and initial pressures (1.0 bar, 1.5 bar, 2.0 bar, 2.5 bar), covering wide range of methanol concentration 5-50 vol. %. The effects of initial conditions on the explosion characteristics were analyzed. The obtained data allows the calculation of the peak pressure at any value of initial pressure even beyond the investigated range (as long as the combustion process reacts as a deflagration), which is important in formulating safety recommendations for process conditions different from the ambient. One of the main conclusions is that in explosions of methanol-air mixtures in vessels, the peak pressures show linear dependency on total initial pressure, at a constant initial temperature and fuel/oxygen ratio. Although the results from the evaluation indicate that presented theoretical simulations can become a valuable tool for rough estimation. On the other hand the modeling requires further improvements to become an useful for consequence modeling and design of industrial facilities. Thus, at the first stage, the equilibrium calculations can be used as a rough calculation of the worst case scenario. At the same time, these values will be used as approximate initial values for explosion experiments carried out in heated 1 m3 and 20 dm3 explosion apparatuses designed by OZM Research s.r.o. and used at Energy Research Centre, VŠB - Technical University of Ostrava. The results represent a continuation of numerous efforts by our research group, where understanding of results obtained in laboratory tests for predicting the consequences of gas/vapor mixtures worst case explosion indices [7-9] is the key underlying problem of interest. In the near future an experimental study on the explosion characteristics of methanol–air explosions in a closed spherical vessel with central ignition will be carried out, using methanol–air mixtures with variable composition, at various initial pressures and temperatures in order to confirm the presented predictions.
Acknowledgement
This work would not have been possible without the financial support of the grant Innovation for Efficiency and Environment - Growth, reg. no. LO1403 supported by National Programme for Sustainability and financed by the Ministry of Education, Youth and Sports.
References
[1] HUANG, Li, Q.; CHENG, Y.; HUANG, Z. Comparative assessment of the explosion characteristics of alcohol-air mixtures. Journal of Loss Prevention in the Process Industries, 2015, roč. 37, s. 91-100. Dostupný také z: http://www.sciencedirect.com/science/article/pii/S0950423015300097. ISSN 0950-4230.
[2] WANG, Liu, H., …[et al.]. Comparative study on alcohol-gasoline and gasoline-alcohol Dual-Fuel Spark Ignition (DFSI) combustion for engine particle number (PN) reduction. Fuel, 2015, roč. 159, č. 9362, s. 250-258. Dostupný také z: http://www.sciencedirect.com/science/article/pii/S0016236115006390. ISSN 0950-4230.
[3] ECKHOFF, R. Explosion Hazards in the Process Industries. 1st Ed. Gulf Publishing Company: 2005. 436 pgs. ISBN 978-0-9765-1134-2.
[4] Center for Chemical Process Safety. Guidelines for Safe Storage and Handling of Reactive Materials. Center for Chemical Process Safety, 1995. 364 s. ISBN 978-0-8169-0629-1.
[5] MOORE, W. J. Physical Chemistry. 4th.ed. Longmans Green & Co, 1963.
[6] REYNOLDS, W.C. The element potential method for chemical equilibrium analysis: implementation in the interactive program STANJAN: version 3 : 1986. Department of mechanical engineering Stanford University, 1986.
[7] SKŘÍNSKÝ, J. …[et al.]. Theoretical analysis of explosion behavior for CO/O2/N2, CO/O2/N2/H2O and CO/O2/N2/CO2 mixtures. Časopis výzkumu a aplikací v profesionální bezpečnosti [online], 2015, roč. 8, č. 4. Dostupný z: http://www.bozpinfo.cz/josra/josra-04-2015/analysis-explosion-behavior.html. ISSN 1803-3687.
[8] SKŘÍNSKÝ, J. …[et al.]. Explosion characteristics of syngas mixtures at elevated temperatures and pressures. Časopis výzkumu a aplikací v profesionální bezpečnosti [online], 2015, roč. 8, č. 4. Dostupný z: http://www.bozpinfo.cz/josra/josra-04-2015/explosion-characteristics.html. ISSN 1803-3687.
[9] SKŘÍNSKÝ, J. …[et al.]. Maximum explosion pressure of H2/O2/N2 mixture at various initial pressures. Časopis výzkumu a aplikací v profesionální bezpečnosti [online], 2015, roč. 8, č. 4. Dostupný z: http://www.bozpinfo.cz/josra/josra-04-2015/maximum-explosion.html. ISSN 1803-3687.
Vzorová citace
SKŘÍNSKÝ, Jan; VEREŠ, Ján. Explosion characteristics of methanol-air mixtures at elevated temperatures and pressures - numerical study. Časopis výzkumu a aplikací v profesionální bezpečnosti [online], 2016, roč. 9, č. 3. Dostupný z: http://www.bozpinfo.cz/josra/explosion-characteristics-methanol-air-mixtures-elevated-temperatures-and-pressures-numerical. ISSN 1803-3687.
Užitečné odkazy
Provozovatel portálu
Jeruzalémská 1283/9
110 00 Praha 1


